Adsorption and Adsorbates
When gas molecules get stuck on surfaces
- It may be reflected,
- it may bind to the surface, remaining intact as a molecule (molecular adsorption), or
- it may dissociate, and its fragments (e.g., the atoms it consists of) bind to the surface (dissociative adsorption).
- In a few special cases, gas molecules can dissociate as they hit a surface, and only part of the fragments remain on the surface, and part of the molecule is abstracted, i.e., leaves the surface (abstractive adsorption).
If the molecule or its fragments are bound to the surface, they are known as adsorbates. When they leave the surface, we speak of desorption. Understanding adsorption and adsorbates is essential for gaining insight into catalysis, e.g. exhaust gas purification by catalysts, where different toxic gases adsorb on the catalyst, react there and the reaction products (non-toxic gases) desorb from the surface.
Dissociative adsorption - hot adatoms?
When an oxygen molecule (O2) hits an aluminum surface, dissociates and the oxygen atoms are bound to the aluminum atoms, a lot of energy is released (coming from the high binding energy between oxygen and aluminum). If all this energy (more 5 eV per O atom) were transferred to the O atoms, they would be really hot, about ten times the temperature of the atoms at the surface of the sun! So even a part of this energy would be sufficient to call the O atoms “hot adatoms”, and people have been wondering what the oxygen atoms do with all this energy …
With these thoughts in mind, and based on STM images interpreted as showing single oxygen atoms widely apart, it was claimed in a famous article [H. Brune et al., Phys. Rev. Lett. 68 (1992) 624] that the hot oxygen atoms “fly apart at least 80 Å before their excess energy is dissipated.” One Ångström (1 Å) is 1/10000000 of a millimeter, or about 1/2 to 1/3 of the size of an atom, so 80 Å is not particularily far by usual standards, but it is quite a lot for an atom: almost 30 times the distance between two Al atoms.
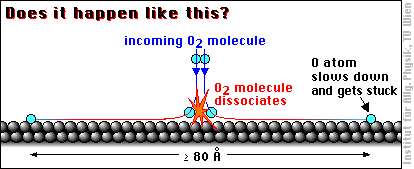
This motion has been called transient mobility, because it occurs only for a very short moment after adsorption.
Theoretical studies triggered by this work could not confirm this result, the Al surface is too bumpy and the oxygen atoms - even if receiving the maximum energy possible - would be slowed down very efficiently. Although there may be some ways out of this dilemma (abstractive adsorption, “cannon-ball” trajectories over the surface) we decided to have a look with our STM - in the meanwhile the state of the art had advanced a bit and we could get somewhat better resolution.
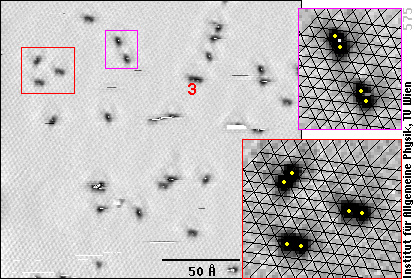
Now, we see the lattice of the aluminum atoms (faint grey texture in the left frame), and the oxygen atoms betray their existence by dark spots. That's because the oxygen atoms themselves don't carry tunneling current, while they suck electrons from the Al atoms. With fewer electrons less tunneling current can flow through the Al, and that's why it looks darker (deeper) at the sites of the oxygen atoms (see the introduction to STM).
If we look carefully, we note that the dark spots are not round, but elongated - so they can't be single oxygen atoms! We have enlarged two parts of the image (red and purple frames) and drawn black lines over the Al atoms - here it becomes obvious that the elongated black spots are due to two oxygen atoms (positions marked as yellow dots), each of them between three Al atoms.
In other words, we see pairs of oxygen atoms, whereas with lower resolution, one could mistake them for single O atoms.
If there were only pairs, everything could be simple: the pairs would simply correspond to the O2 molecules. In the STM images, we also see groups of three (or more) oxygen atoms, however, one of them marked with a “3” in the image above. But we certainly did not have any ozone (O3) in the gas!
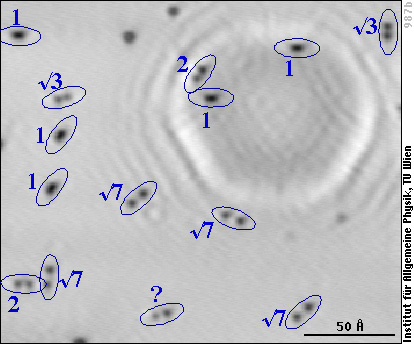
The solution to the puzzle finally came from STM measurements with the aluminum surface at low temperature. Whereas this should not affect the transient mobility, at low temperature (-190 °C) we can be sure that the atoms don't move (diffuse) after they have lost the adsorption energy. And that's what the surface looks like after oxygen has been adsorbed at room temperature:
The aluminum lattice is not resolved, but we see the individual oxygen atoms as dark spots as in the previous image, and we note that they are either elongated (as the pairs of O atoms in the previous image) or come in pairs of two clearly separated O atoms. We have labelled each pair with the distance between the two O atoms, given in multiples of the distance between two aluminum atoms (“1” stands for 2.86 Å, “2” for 5.72 Å, etc.). In our experiments we hardly ever observe a distance above 7.6 Å (“√7” in the image), far below the 80 Å distance claimed in the study mentioned above.
Summing up all these and some more of our results, we can now understand what happens when O2 adsorbs on aluminum: After dissociation, the two oxygen atoms can either land next to each other (resulting in the pairs marked “1” in the low-temperature image) or part of the adsorption energy can allow them to jump over a few aluminum atoms before they lose all their energy. At -190°C the atoms remain there. At room temperature, single oxygen adatoms are mobile and diffuse over the surface until they either form a stable pair with the second atom of the molecule they originate from or they can attach to a pre-exisiting pair (or larger group) and form a group of three (or more) oxygen atoms on the surface. Pairs and larger groups of oxygen adatoms do not move any more at room temperature.
So it is quite a long story what can happen when one oxygen molecule hits a pure metal surface and starts to oxidize it - imagine how much more can happen on more complex surfaces…
Adsorption on alloys - where the adsorbates want to sit
Today, catalysts are not only used for exhaust gas purification but also for many processes in chemistry. Alloys often perform better in catalysis than pure metals, and that's why many catalysts are based on alloys.
At a first view, if we have an alloy, we can expect that the atoms in an alloy simply behave as usual, so a silver atom in a silver-palladium alloy behaves like the noble metal silver, while the palladium atoms in the alloy are more reactive, as we know it for the metal palladium. This means that adsorption on the alloy is simply governed by chemical affinity.
If we adsorb oxygen on a silver-palladium (AgPd) alloy surface, we find this kind of behaviour, indeed:

What you see here is the alloy surface, consisting of 95% silver atoms (individual atoms not resolved under these tunneling conditions) and about 5% palladium atoms visible as white dots (this is a case of chemical contrast). The black dots are oxygen atoms (from dissociative adsorption of the oxygen molecules). As these black dots appear as large as the Pd atoms, we cannot directly determine whether the oxygen atoms bind to Pd atoms on the surface or not.
Sometimes, the oxygen atoms can hop (diffuse) from one place to another. The right image was taken 20 seconds after the left one, and you may notice that some black oxygen atoms have changed their position (red arrows). The site an oxygen atom leaves always appears bright thereafter, so there is a palladium atom. Similarily, the new position an oxygen atom takes is always one that has been bright before, i.e., at a Pd atom. This means that the oxygen atoms are strongly bound to palladium atoms, and remain there for a while, but they have much lower chemical affinity to silver atoms and thus don't remain on the pure silver surface for long.
Adsorption on alloys is not as simple as the AgPd example may suggest - the atoms in an alloy do not always behave the same way they would behave in the respective pure metal. The chemical properties of an atom are influenced by its environment. For example, a platinum atom in a platinum-cobalt (PtCo) alloy does not behave like a platinum atom in pure platinum. This effect is known as ligand effect - the word “ligand” stands for the “neighbor”, i.e., a neighboring atom in the alloy.
To see what this means, let us analyze these two STM images:
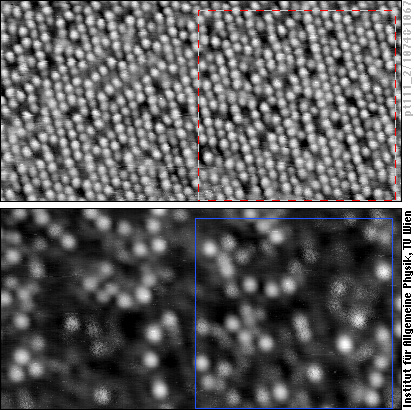
The upper image shows a PtCo alloy with about 80% Pt atoms (looking like white balls) and 20% Co atoms (appearing grey or black and more blurred than the Pt atoms). At this surface carbon monoxide (CO) molecules have adsorbed. The lower STM image shows the same area of the surface, but here the tunneling conditions have been set to show the CO molecules (white).
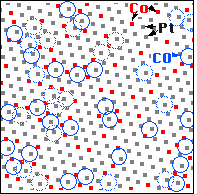
We can now superimpose these two images on the computer and make a map to see where the CO molecules are bound - we have done this for the framed area in the STM images. We first note that the CO molecules (blue circles) are always on top of one metal atom (square dot in the map), not in a “bridge site” between two or a “hollow site” between three metal atoms. Second, CO molecules are only on top of Platinum atoms (white balls in the STM image, grey dots in the map), never on top of cobalt atoms (red dots in the map). Up to now, what we have observed are the properties of the pure metals platinum and cobalt: CO molecules sits on top of these atoms and bind more strongly to Pt than to cobalt.
We come to the third observation when we look at the surroundings of the Pt atoms that CO molecules bind to: CO never binds to a Pt atom having only Pt neighbors! If one counts how many CO molecules are adsorbed on Pt atoms with one, two, … cobalt neighbors in the surface, we find that Pt atoms with more cobalt neighbors are more likely to be the binding site of a CO molecule than Pt atoms with fewer cobalt neighbors. This is a clear demonstration of the ligand effect - showing that the properties of an atom are modified by its neighbors.
There is much more that we can learn from our STM images, e.g.,
- Some CO molecules (dotted in the map above) appear mottled or fuzzy in the STM images, showing us that they don't stay where they are but rather jump between different sites quite rapidly (in less than 1/1000 of a second).
- CO bonding on the alloy in general is much weaker than on either pure metal, platinum or cobalt.
- Binding of CO molecules is stronger on Pt atoms which are sitting just a tiny bit higher than the others.
- And more, but we don't want to risk that you become tired before you have seen all of our STM Gallery!
For further information
- M. Schmid, G. Leonardelli, R. Tscheließnig, A. Biedermann and P. Varga
Oxygen adsorption on Al(111): low transient mobility
Surf. Sci. 478 (2001) L355-L362. Full text*
- P. T. Wouda, M. Schmid, B. E. Nieuwenhuys, and P. Varga
Adsorbate migration on PdAg(111)
Surf. Sci. 423 (1999) L229-L235. Full text*
- Y. Gauthier, M. Schmid, S. Padovani, E. Lundgren, V. Bus, G. Kresse, J. Redinger, P. Varga
Adsorption sites and ligand effect for CO on an alloy surface: a direct view
Phys. Rev. Lett. 87 (2001) 036103. Full text
* Please note: access to full text (PDF files) of some articles is restricted due to copyright reasons.
The typos and other errors on this page are are caused by: Michael Schmid ([email address: lastname @ this server · enable javascript to see it]).