Surface Reconstructions
Rearrangement of atoms at a surface
Since the bonding geometry of atoms at the surface differs from that in the bulk, on some surfaces the atoms rearrange and form a structure energetically more favourable than the truncated bulk. In surface physics, such a rearrangement is called reconstruction.
The way to the platinum 'hex' reconstruction
Among the metals, the 5d-elements iridium, platinum and gold show a strong tendency towards reconstruction, i.e., most or all of their low-index faces are reconstructed. The surfaces of the 3d and 4d elements (in the periodic table the rows above Ir, Pt and Au) remain unreconstructed. So we can gradually increase the tendency towards reconstruction by alloying the 3d-element nickel and the 5d-element platinum and look what the surface looks like with increasing Pt concentration.
Whereas the (100)-oriented surface of Ni is a simple square lattice of atoms, Pt(100) reconstructs, having one close-packed (pseudohexagonal, 'hex') layer of atoms on top of the square lattice of the unreconstructed second layer. And this is what the (100) surface of a PtNi alloy looks like at approx. 68% Pt concentration in the first monolayer:


What you see in most of the image are single bright rows of atoms shifted by half an interatomic distance in the direction of the rows (red arrow). You will also notice that these atoms have a hexagonal environment (6 nearest neighbours) in the first monolayer, which is already the same local structure as in the 'hex' reconstruction of pure Pt. Since the shifted row atoms do not sit in the the hollow sites between four atoms of the second layer, but rather in bridge sites between two second-layer atoms, they are higher and thus brighter in the STM image. The black patches in the image are due to an impurity (segregated carbon).
In the upper right corner you can also see two stripes of the 'hex' reconstruction. There, the close-packed surface layer has a higher atom density than the square lattice below, i.e., 8 surface atoms per 7 substrate atoms. Some surface atoms sit on top of substrate atoms, some in bridge sites and some in the the hollow sites between four atoms of the second layer, and thus lower. You can see these height differences as different brightness of the atoms in the STM image.
With higher platinum concentration the whole surface layer becomes pseudohexagonal - already similar to the (100)-oriented surface of pure Pt:
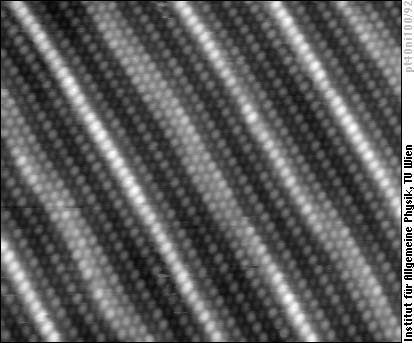
Whereas the above image shows only the geometrical structure, we can also obtain an image showing a superposition of geometry and chemical contrast. In this image the Ni atoms appear as bright blobs, whereas Pt atoms in the same row (same geometric height) are darker:
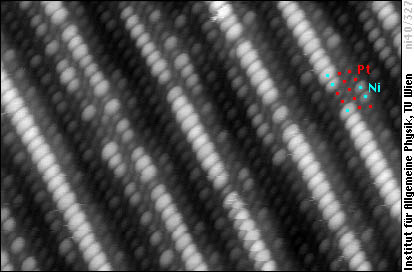
It is obvious that more Ni atoms (bright blobs) are found in the higher positions, especially in the sites on top of second-layer atoms, where the coordination (number of nearest neighbours) is lowest. Platinum prefers sites with higher coordination, as there are only a few bright Ni atoms in the lower rows.
An important point is the driving force of the reconstruction, i.e., the question ”why does Pt reconstruct?”. A detailed analysis of the reconstructed alloy surfaces shows that the reconstruction cannot be due to tensile stress in the surface, which was often believed to be the driving force of the 'hex' reconstruction. Since the 'hex' reconstruction reduces the number of surface atoms with the lowest coordination number, it rather seems plausible that the tendency of Pt to avoid low-coordinated sites, as seen in the image above, plays an important role.
Adsorbate/segregand-induced reconstructions
Reconstructions can be also induced by atoms adsorbed (from the gas phase) or segregated (from the bulk) to the surface. Pure PtRh alloy surfaces with (100) orientation are - in contrast to PtNi shown above - unreconstructed. When oxygen is adsorbed on such a surface, the individual oxygen atoms first take positions in so-called fourfold hollow sites, i.e., in between four of the metal atoms. You can see some of these O atoms as small bright spots in the lower half of the image below (two of them are marked with yellow arrows).
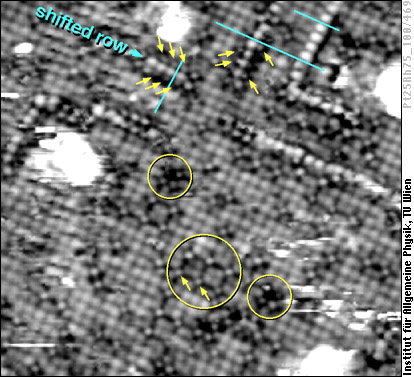
With higher oxygen concentration, the oxygen atoms could in principle occupy all the fourfold hollow sites, but this seems to be an unfavorable situation. Only in a few cases we found single metal atoms surrounded by four oxygen atoms (small yellow circles; the metal atoms appear too dark to be visible there). More often we find the O atoms to keep some distance, such as in the larger yellow circle. As more oxygen is added, what rather happens is a reconstruction of the PtRh alloy surface. Single shifted rows of metal atoms are formed, similar to those discussed above. You can see this by help of the aqua-green lines in the STM image, running on-top of all metal atoms except the bright atoms in the shifted rows. In the oxygen-induced reconstruction, the shifted rows are flanked by oxygen atoms, which are barely visible next to the shifted rows in some places (yellow arrows).
At higher oxygen coverages (especially at elevated temperatures), the oxygen-induced shifted row reconstruction covers all of the surface, and each third row becomes a shifted row. It has been speculated that this very structure, which can easily uptake and free oxygen, is one of the features important for the good performance of platinum-rhodium catalysts (the most common catalysts for car exhaust gases).
Now moving towards segregand-induced reconstructions, let us have a look at chromium, a metal widely used due to its high resistance to corrosion. The pure and unreconstructed (110) surface of chromium has a roughly hexagonal arrangement of atoms at the surface, whereas nitrogen leads to several different reconstructions. Among them is the “herringbone” reconstruction shown in this STM image:
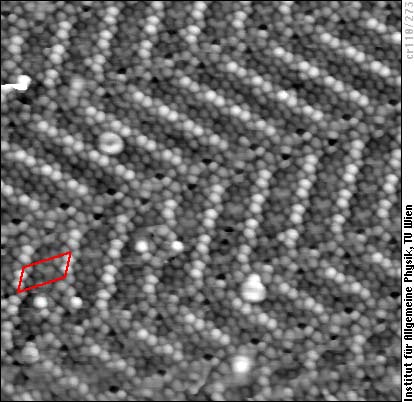
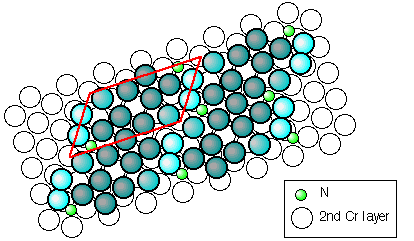
The STM image shows only the Cr atoms of the first layer, the positions of the nitrogen atoms and the Cr atoms in the second layer can be determined only indirectly. Counting the atoms in the oblique unit cell (marked red), one can easily verify that there are 11 Cr atoms per cell in the first monolayer, but 12 of them in the second and all other layers below. It is also easy to see that the N atoms do not sit between close-packed Cr atoms, but rather in a those places where most space is available. We therefore conclude that this reconstruction takes place to make more space for the N atoms by removing one Cr atom per cell from the first layer. Actually, this is one of the most frequent driving forces (reasons) for segregand-induced or adsorbate-induced reconstructions.
For further information
The 'hex' reconstruction of pure Pt(100)
- A. Borg, A.-M. Hilmen, and E. Bergene
STM studies of clean, CO- and O2-exposed Pt(100)-hex-R0.7°
Surf. Sci. 306 (1994) 10-20.
- G. Ritz, M. Schmid, P. Varga, A. Borg, and M. Rønning
The Pt(100) quasihexagonal reconstruction: A comparison between scanning tunneling microscopy data and effective medium theory simulation calculations
Phys. Rev. B 56 (1997) 10518-10525. Full text
- …and dozens of other articles, some of them cited in the two mentioned above.
PtNi(100) - from shifted rows to 'hex'
- M. Schmid, A. Biedermann, S. D. Böhmig, P. Weigand and P. Varga
The shifted row reconstruction of PtxNi1-x(100)
Surf. Sci. 318 (1994) 289-298.
- W. Hebenstreit, G. Ritz, M. Schmid, A. Biedermann and P. Varga
Segregation and reconstructions of PtxNi1-x(100)
Surf. Sci. 388 (1997) 150-161. Full text*
Oxygen-induced shifted-row reconstruction of PtRh(100)
- P.T. Wouda, M. Schmid, W. Hebenstreit, and P. Varga
Interaction of oxygen with PtRh(100) studied with STM
Surf. Sci. 388 (1997) 63-70. Full text*
- M. Sporn, E. Platzgummer, E.L.D. Gruber, M. Schmid, W. Hofer and P. Varga
A quantitative LEED analysis of the oxygen-induced p(3×1) reconstruction of Pt25Rh75(100)
Surf. Sci. 416 (1998) 384-395. Full text*
Cr(110) - the herringbones
- M. Schmid, M. Pinczolits, W. Hebenstreit, and P. Varga
The nitrogen-induced herringbone reconstruction of Cr(110)
Surf. Sci. 389 (1997) L1140-L1146. Full text*
* Please note: access to full text (PDF files) of some articles is restricted due to copyright reasons.
The one who has made all the typos and other errors on this page: Michael Schmid ([email address: lastname @ this server · enable javascript to see it]).