Chemical Contrast in STM
Surface chemical order on alloys
The surface of alloys may differ in several respects from the corresponding bulk structure. First of all, the composition of the surface usually differs from the bulk, but the surface may also exhibit a chemical order different from the one in the bulk of the material. This influences processes such as gas adsorption and catalysis, since adsorbed molecules often prefer a certain chemical environment of substrate atoms.
It all started with an (111) oriented surface of a PtNi alloy (bulk composition 25% Pt, 75% Ni; surface approx. 50% of each). Quite unexpectedly at that time, we found that we can distinguish between Pt and Ni atoms on this surface with the STM! What we saw was an arrangement with small domains of alternating chains of Pt and Ni atoms:
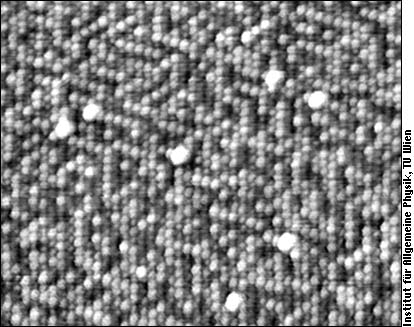
Since Pt and Ni are about 50% of the surface atoms each, we cannot directly determine which species is white and which dark. Using other images with chemical contrast of PtNi we could find out that the brighter species is Ni. The white blobs in the image are impurities of unknown nature.
Our first STM images with chemical contrast on PtRh(100) have been obtained at our institute, as result of a cooperation with P. T. Wouda and B. E. Nieuwenhuys (Leiden University, Netherlands).
The surface structure of PtRh is especially interesting since PtRh alloys are used for catalysts for exhaust gas of car engines. The surface is where catalysis happens!
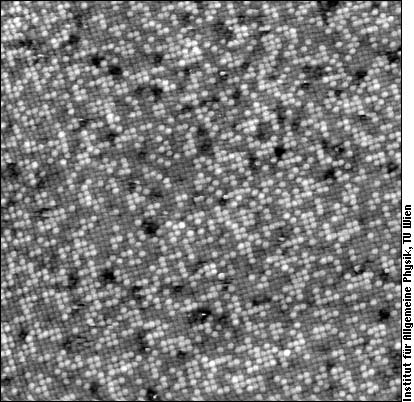
Here, the bulk contains 50% of both elements, but there are approximately 31% Rh (bright) and 69% Pt atoms (darker) at the surface. The black spots are caused by residual carbon. The atoms of Pt and Rh tend to cluster in small groups of the same species.
As you can see in the following image, a step edge on PtRh(100) is mainly populated by the darker Pt atoms.
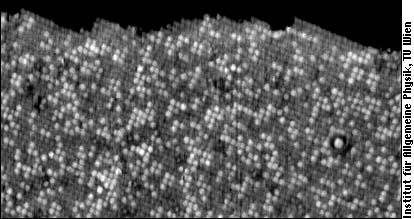
As mentioned, the Rh atoms tend to form small groups (“clusters”) on the PtRh(100) surfaces shown above. In other words, Rh atoms tend to have more Rh neighbors than in a pure random distribution, and Pt atoms tend to have Pt rather than Rh neighbors. PtRh alloys are, however, just at the very edge between preference for clustering (demixing) and preference for unlike nearest neighbors. Depending on the sample preparation, one can actually get one or the other with the same Pt25Rh75(100) crystal. It is therefore not astonishing that a search for literature we recently did turned up three articles claiming that PtRh bulk alloys prefer demixing, and three others claiming the opposite! This is not a purely academic question - for catalysis it is necessary to understand gas adsorption, and surface order can severely influence the number of available sites. Just imagine an atom like oxygen, which prefers sitting between four neighboring Rh atoms. It will find plenty of such sites on the surface shown above, but hardly any favorable site if Rh clusters are avoided.
On PtRh(111) surfaces, as on most surfaces of Pt alloys with (111) orientation, we see short chains of atoms of the same species (a bit similar to PtNi(111) above):
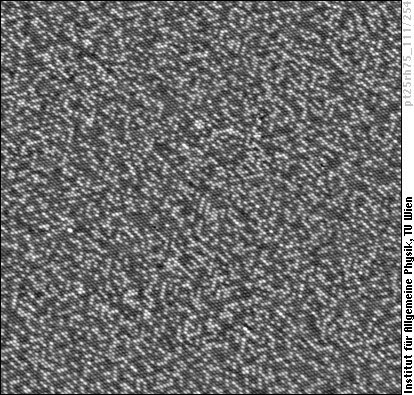
We have squeezed quite a lot of atoms (more than 14000) into this small frame, but you may also enjoy the full splendor of this image in a bigger view.
Mentioning the number of atoms, we should also say that STM images with chemical contrast allow to determine the chemical composition of the surface layer by simple atom counting - in this case 31% Rh (bright) and 69% Pt (darker) atoms. It's nice to see that this agrees well (within 2%) with other methods of surface analysis, such as low energy ion scattering (LEIS) and quantitative low energy electron diffraction (tensor-LEED). As the bulk of the crystal consists of 75% Rh and 25% Pt, this case again shows that bulk and surface can be quite different.
Coming back to the topic of surface chemical ordering, here is a fine example for a surface where unlike chemical species are preferred as nearest neighbors (the opposite to demixing). On a PtCo(100) surface almost each Pt atom (bright) is surrounded by Co atoms only, and vice versa, but you won't recognize this at once when looking at the image:
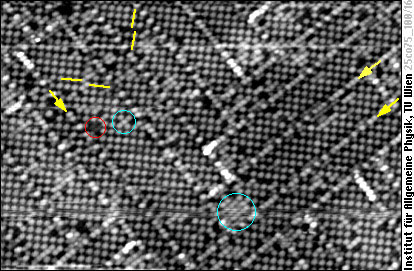
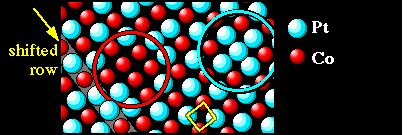
In this image, chemical contrast is so strong that only the Pt atoms are visible as protrusions, whereas the Co atoms remain invisible. Apart from a few shifted rows (some marked by yellow arrows), the metal lattice at the surface is a square lattice (yellow square in the model). Usually Pt occupies every other lattice site, so the Pt atoms again form a square lattice, but rotated 45° with respect to the metal lattice. In some places additional Pt atoms appear in sites usually occupied by Co (aqua-green circles), but also Co atoms can sit in Pt sites (red circle). Further imperfections of the “checkerboard” chemical order are antiphase domains, i.e., regions with the Pt and Co sublattices exchanged. This is shown by yellow lines in the STM image, marking the Pt rows on each side of the antiphase domain bondaries.
For further information
- An overview article on alloy surfaces viewed by STM
M. Schmid and P. Varga
Segregation and surface chemical ordering - an experimental view on the atomic scale
In: The Chemical Physics of Solid Surfaces, Volume 10: Alloy surfaces and surface alloys; ed. by. D. P. Woodruff, Elsevier 2002, ISBN 0-444-51152-0. Full text*- It all started with PtNi(111)
M. Schmid, H. Stadler and P. Varga
Direct observation of surface chemical order by scanning tunneling microscopy
Phys. Rev. Lett. 70 (1993) 1441-1444. Full text- PtRh surfaces
E.L.D. Hebenstreit, W. Hebenstreit, M. Schmid, and P. Varga
Pt25Rh75(111), (110), and (100) studied by scanning tunneling microscopy with chemical contrast
Surf. Sci. 441 (1999) 441-453. Full text*P. T. Wouda, B. E. Nieuwenhuys, M. Schmid and P. Varga
Chemically resolved STM on a PtRh(100) surface
Surf. Sci. 359 (1996) 17-22.- PtCo(100)
Y. Gauthier, P. Dolle, R. Baudoing-Savois, W. Hebenstreit, E. Platzgummer, M. Schmid, P. Varga
Chemical ordening and reconstruction of Pt25Co75(100): a LEED/STM study
Surf. Sci. 396 (1998) 137-155. Full text*
* Please note: access to full text (PDF files) of some articles is restricted due to copyright reasons.
The typos and other errors on this page are due to the inattentiveness of Michael Schmid ([email address: lastname @ this server · enable javascript to see it]).


