Non-metals, too!
Oxides and grains of salt…
As a scanning tunneling microscope uses an electrical current flowing between a fine tip and the sample (see the introduction to STM), an STM works on electrically conducting surfaces only. Nevertheless, we can use it to study materials like oxides or ionic crystals in certain cases. Whereas some oxides are conducting electricity sufficiently well, materials like NaCl (common salt) are usually very good insulators. We can use the STM to “see” them only if they are very, very thin - one or two layers of atoms.
Grains of salt
Common salt, also known as rocksalt or sodium chloride (NaCl), is a well-known ionic crystal. As mentioned, it is a very good insulator; nevertheless we can get images of ultra-thin NaCl films grown on a metal. The tiny NaCl crystals observed by us usually have rectangular shapes, similar to the larger crystals in everyone's kitchen. But you would need a few billions of billions of the crystals shown below for a bowl of soup!
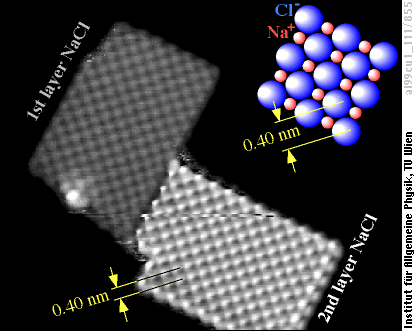
What you see here is just the NaCl crystal, the aluminum supporting it is shown black. The distance between the atoms seen in the NaCl island is 0.4 nanometers. This means that we see only one type of atom, Na or Cl, as comparison with the well-known structure of NaCl (top right) shows. Based on the STM images we can't decide which type of atoms we observe. Calculations show that chlorine appears bright (as a protrusion) in the STM images, because it “borrows” electrons from the supporting aluminum.
Sodium chloride crystals found in nature usually have a nice cubic shape, and even if the shapes are more irregular, all faces have so-called {100}-orientation, i.e., parallel to the sides of the cube. Also the tiny NaCl crystal in the image above is bounded by {100}-oriented faces on all its sides, and also the top surface is {100}-oriented. If you cut away one of the corners of a cube, this would be a {111} face. But one cannot cut an NaCl crystal cube in this direction, and if one tries to create such a surface, e.g., by grinding and polishing, it will usually form microscopic {100}-oriented faces. The reason is rather simple: NaCl{100} faces are electrically neutral, while {111} faces are not - they would consist of either Na (positive ions) or Cl (negative ions). Such a polar surface would cause a huge electric field, associated with high energy, a very unfavorable situation.
Nevertheless, we have found out that it is all different for ultra-thin films: By adding Na to one-layer thin NaCl islands, we get STM images like this:
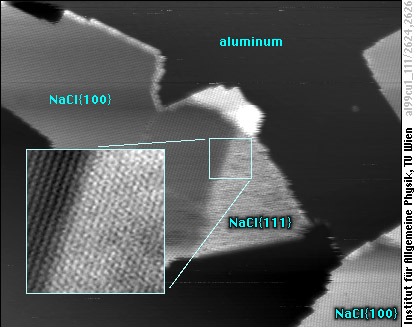
Besides the usual {100} surface of NaCl, we now observe the one of the most unusual crystals of sodium chloride in the world, with triangular rather than rectangular shape, and also a different arrangement of the atoms (no square lattice any more).
What has happened? We have converted part of the usual {100}-oriented islands to a {111} surface! Whereas the structure of this new surface is that of NaCl{111}, the chemical composition is Na2Cl. The newly formed NaCl{111} area consists of an Na layer below, then a Cl layer, and finally an Na layer on top. Calculations show that this structure is stable as an ultra-thin film because the positive ions (Na+) carry only half the charge they would have in a normal NaCl crystal, thus keeping the Na2Cl structure electrically neutral.
Oxides
Many oxides are interesting for catalysis - they can be catalysts by themselves, they can store oxygen needed for catalytic reactions on some other surfaces, or they are used as a support for fine metal particles. “Support” does not necessarily mean that the oxides have a passive role only, they may strongly enhance or modify the catalytic reactions (a phenomenon known as strong metal support interaction). And remember: catalysis happens at the surface, so we have to study the surface to understand catalysis!
One promising candidate for future exhaust gas catalytic converters is the metal ruthenium, as it forms an oxide (RuO2) having high efficiency in converting the poisonous CO (carbon monoxide) to the comparatively harmless CO2 (carbon dioxide). We have studied this conversion in collaboration with H. Over and G. Ertl (Fritz-Haber-Institut, Berlin).
RuO2 has rutile structure, and its most stable surface ({110} orientation) is shown in the model below:
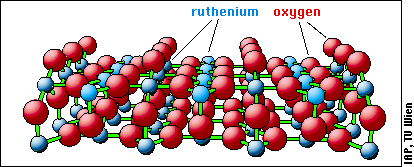
The surface consists of rows of oxygen and ruthenium atoms, and calculations indicate that STM should show the rows of protruding oxygen atoms. The first STM image (below) shows what such a RuO2 surface created by oxidation of a Ru surface looks like:

The second image (above) shows what the same surface looks like after exposure to a small amount of CO at 75 °C. In spite of the somewhat lower resolution of this image it is clear that CO has created a lot of defects - each CO molecule needs one oxygen atom to form CO2, and these oxygen atoms are simply taken from the RuO2 surface. When exposing the surface to more CO at higher temperatures, all oxygen in the whole RuO2 surface is consumed, and finally only metallic Ru remains on the surface. Additional oxygen is needed to oxidize the ruthenium again. For a catalytic converter therefore some oxygen must be present in the exhaust gas (also in today's cars, which do not use Ru catalysts yet).
For further information
- Sodium chloride, seen by STM
W. Hebenstreit, J. Redinger, Z. Horozova, M. Schmid, R. Podloucky, P. Varga
Atomic resolution by STM on ultra-thin films of alkali halides: experiment and local density calculations
Surf. Sci. 424 (1999) L321-L328. Full text*- The unusual NaCl{111} surface
W. Hebenstreit, M. Schmid, J. Redinger, R. Podloucky, P. Varga
Bulk terminated NaCl(111) on aluminum: A polar surface of an ionic crystal?
Phys. Rev. Lett. 85 (2000), 5376-5379. Full text- CO -> CO2 conversion on RuO2
H. Over, Y.D. Kim, A.P. Seitsonen, S. Wendt, E. Lundgren, M. Schmid, P. Varga, A. Morgante, G. Ertl
Structure and catalytic reactivity on atomic scale of an oxide surface: RuO2(110)
Science 287 (2000) 1474-1476. Full text*H. Over, A.P. Seitsonen, E. Lundgren, M. Schmid, P. Varga
Direct imaging of catalytically important processes in the oxidation of CO over RuO2(110)
J. Am. Chem. Soc. 123 (2001) 11807-11808. Full text*
* Please note: access to full text (PDF files) of some articles is restricted due to copyright reasons.
Responsible for errors on this page:
Michael Schmid, IAP/TU Wien ([email address: lastname @ this server · enable javascript to see it]).


